home
NACA coordinates HIV activities in Nigeria
Facilitate the development and management of the policies and strategies of all sectors to ensure the human, financial and organizational resources to support the successful execution of the national HIV/AIDS response programme.

DG NACA speaks
on the forthcoming Nigeria HIV Prevention Conference 2024

Advocacy For
Intervention
NACA works to provide an enabling policy environment and stable ongoing facilitation of proactive multi sectoral planning, coordinated implementation, monitoring and evaluation of all HIV/AIDS prevention and impact mitigation activities in Nigeria
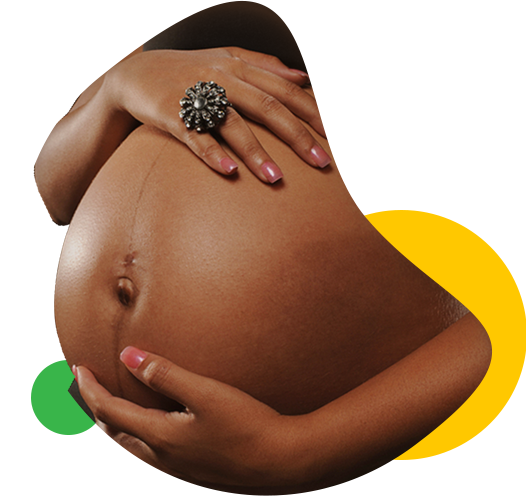
Prevention of mother-child transmission of HIV/AIDS is possible
HIV medicines reduce the amount of HIV in the body. Having less HIV in the body reduces a woman’s risk of passing HIV to her child during pregnancy and childbirth. Having less HIV in the body also protects the woman’s health.
Some of the HIV medicine passes from the pregnant woman to her unborn baby across the placenta (also called the afterbirth). This transfer of HIV medicine protects the baby from HIV infection
Latest News
“HIV does not make people dangerous to know, so you can shake their hands and give them a hug: Heaven knows they need it”.
Diana, Princess of Wales

Your One Stop Information HIV/AIDS related Disease
You can call Our emergency toll free line









































